Talks will be arranged into three categories:
1. Key methodologies for different applications. Discussion of whether these methodologies are transferable, and how they can be shared.
2. Specific examples of biomedical applications and diseases studied using multi-scale methods and combining different methodologies. Discussion of how this can be extended to other diseases.
3. Best practices for establishing collaborations with clinicians including development of common language, exchange of data, and application of predictive simulations. Discussion of how to assemble, adopt, and disseminate best practices.
Suggestions of additional topics or extensions of the initially suggested topics will be solicited from the members of the Multi-scale Systems Biology WG and from other members of the MSM Consortium. In the lead-up to the conference, we will organize several webinars to discuss and refine visions for specific conference tracks. These webinars will help to identify and discuss central topics of importance in specific fields and tracks in advance of the conference, so that the conference itself will have well-defined goals for discussions and for outputs of those discussions.
After the conference, a series of webinars will be similarly organized to finalize white papers, reviews, commentaries and other work products of the conference.
Confirmed Invited Speakers and Their Titles/Abstracts
Gary An
Department of Surgery
University of Chicago School of Medicine
"Re-examining the evaluation and use of agent-based models to address the Crisis of Reproducibility, the Translational Dilemma and Precision Medicine"
Agent-based models are increasingly used for the computational representation of biological systems, most often as interacting aggregations of different types of cells. The benefit of agent-based models (ABMs) is that they represent an intuitive way for bioscientists to translate their mechanistic knowledge into computational form. However, their computational complexity does not generally allow the use of more powerful mathematical tools for their analysis, with the result that ABMs are very often treated as in silico experimental objects, with limitations similar to that of biological experimental models, but hampered by being held to the same rigorous validation criteria as simulations from the physical sciences (which emphasize precision of prediction). The key difference is that the physical sciences are governed and constrained by known natural laws, while biology is not (except for evolution). Therefore, existing approaches to evaluating and using computer simulation/mathematical modeling may not be suited to the investigation of biology. This talk will present the argument that the key to biology is its ability to generate heterogeneity at the system level from a shared, common functional structure, manifesting as biological objects’ existence as parameter spaces of conserved functional forms, and that high-performance computing implementations of ABMs can be used a proxy systems to characterize the dynamic extent, or “behavior space,” of biological behavior. This concept of biological systems and the associated use of computational proxy models offers the promise of addressing several major challenges facing bioscience today: 1) The Crisis of Reproducibility, where experimental and clinical studies cannot be replicated, 2) the Translational Dilemma, which is the gap between knowledge generated at the bench and its implementation in effective clinical therapeutics, 3) and Precision Medicine, which should mean the right drug for the right patient at the right time, but is hampered by poor characterization of pathophysiological dynamics.
Daniel Beard
Department of Molecular and Integrative Physiology
University of Michigan Medical School
"Computational systems analysis to predict and analyze targets for improving mechanicalenergetic coupling in the myocardium in heart failure”
The energetic status of the myocardium is compromised in decompensated hypertrophy in the failing heart, with the chemical energy (in the form of the ATP hydrolysis potential) available for the heart to do work diminished compared to normal. Using multi-scale computer models to interpret data from humans and animal models of cardiac decompensation and heart failure, we have developed two novel hypotheses to guide our investigations of how the biochemical/metabolic state of the heart in heart failure affects the mechanical pumping of the heart: (1.) Diminished cytosolic ATP and increased inorganic phosphate (associated with impaired energy metabolism and depletion of cytoplasmic adenine nucleotides) impairs the mechanical function of the heart; and (2.) By blocking purine degradation pathways that may be overactive in the chronically stressed and/or periodically ischemic myocardium, we can increase/restore the nucleotide pool and protect the heart against mechanical dysfunction and failure. Testing these hypotheses using a combination of genetic and surgical models, and computer models, our studies point to the potential promise of whole new classes of pharmacological targets associated with purine nucleotide dephosphorylation, deamination, degradation, and transport.
- https://www.ncbi.nlm.nih.gov/pubmed/19357309
- https://www.ncbi.nlm.nih.gov/pubmed/26910433
- https://www.ncbi.nlm.nih.gov/pubmed/27085901
Danny Bluestein
Biomedical Engineering Department
Stony Brook University
"A Predictive Multiscale Model for Simulating Platelets Activation and Aggregation in Shear Flows"
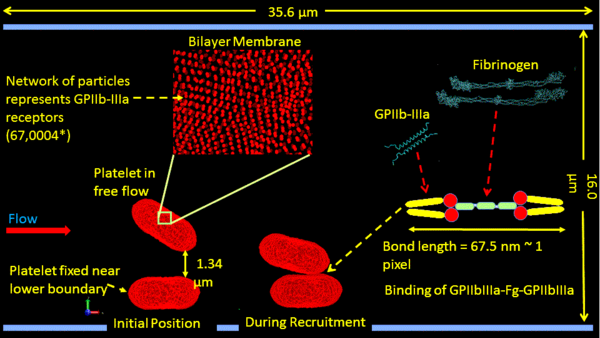
Introduction: One of the early hallmarks of clot formation in prosthetic cardiovascular devices and arterial disease processes is recruitment of platelets to foreign surfaces and aggregate formation in the free flow. Aggregation processes initiate with interaction between quiescent or partially activated platelets through protein receptors and ligands. Continuum methods fail to capture this molecular-scale mechanism. Utilizing molecular dynamics (MD) to model this is computationally prohibitive. We expanded a plate activation multiscale model which interfaces nanoscale microstructures of platelets and microscale transport of blood flows, for providing a more accurate flow induced dynamic stress mapping on platelets and predict aggregation [1-4].
Materials and Methods:
Our multiple spatiotemporal model employs a modified dissipative particle dynamics (DPD) to describe viscous blood flows in the case of stenosis; a coarse-grained MD (CGMD) to describe the platelet membrane, the cytoplasm and the cytoskeleton. CGMD is composed of nonbonded potentials (Lennard-Jones and Morse) and bonded potentials (bonds, angles and dihedrals). Spatially, the DPD-CGMD interface was established by imposing a hybrid force field [1]. Activation-independent platelet recruitment and aggregation were simulated using a Morse-Hooke’s potential (Fig. 1). A novel 4-level multiple time-stepping (MTS) scheme [2] was expanded to improve computing time and accuracy of inter-platelet interactions. The model was validated in vitro by DIC imaging of recruitment of quiescent platelets to platelets adhered on a vWF-coated microchannel for shear stresses of 1-10 dyne/cm2 (100× oil immersion microscopy, 200 fps). Images of platelet attachment were processed in MATLAB to determine geometric parameters of adhered platelets (i.e. circularity, surface area, aspect ratio, and contact area). A feedforward neural network was developed to utilize these geometric parameters and flow conditions to predict inter-platelet contact area.
Figure 1. Multicale model for simulating platelet aggregation in shear flow
Results and Discussion: The model describes the biophysical properties of platelets down to the nm-length and ps-time scales. Membrane Young’s modulus is 31.2 µN/m and shear elastic modulus is 33.0±9.0 µN/m. The model was employed to simulate flow-induced platelet activation [3]. Dynamic stresses of flowing and flipping platelets were mapped on membrane and transmitted to the cytoskeleton. The platelets activated if stress accumulation exceeded their activation threshold. Filopodial formation was achieved via growth of corresponding actin filaments in the highest stress accumulation regions. Most platelets formed 2-3 filopods, with mean length of 1.29±0.17 μm and circularity of 0.99±0.01. Corresponding in vitro experiments in the Hemodynamic Shearing Device (HSD) with subsequent scanning electron microscopy imaging correlated well with the numerical predictions. In addition, aggregation of two platelets was simulated under a wall shear stress of 10 dyne/cm2. The contact time was 0.2-1.0 ms, with Morse parameter α=1.25 yielding contact area of 1.18 µm2 (Fig. 1), in agreement with in vitro aggregation experiments, with a contact area of 1.29±0.48 μm2 (n=20).
Conclusions: Our approach is the first computationally affordable numerical method for simulating platelet activation and aggregation by highly resolved mapping of stresses on the platelet membrane and their transduction to the cytoskeleton under dynamic flow. The blood flow is described at sub mm-length and ns-time scales, while platelet properties are accurately described to nm-length and ps-time scales. Filopodia formation and platelet aggregation were successfully simulated and correlated well with in vitro measurements.
Acknowledgements: This project was funded by NHLBI 1U01HL131052 (DB), NHLBI R21 HL096930-01A2 (DB), and NIBIB Quantum U01EB012487 (DB) and used the XSEDE computer resource award on TACC Stampede (TG-DMS140019, PZ).
References: [1] Zhang, P., et al, Cell Mol Bioeng, 7:552-574, 2014. [2] Zhang, P., et al, J Comput Phys, 284:668-686, 2015.
[3] Pothapragada, S., et al, Int J Numer Meth Biomed Engng, 31:1-16, 2015. [4] Zhang, N. et al, J Comput Phys, 257:726-736, 2014.
Sunny Canic
Department of Mathematics
University of Houston
“Methodologies to study fluid-structure interaction with mesh- and fiber-reinforced biological structures”
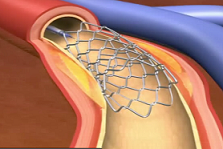
With the recent developments of new technologies, biomedical engineering and medicine, the need for new mathematical and numerical methodologies to aid these developments has never been greater. This talk will outline a new approach to modeling fluid-structure interaction between incompressible viscous fluids such as blood, and mesh-supported or fiber-reinforced biological structures such as arterial walls. The approach is based on dimension reduction to model mesh-like devices such as vascular stents, as one-dimensional PDEs defined on a graph.
Stent in artery
These are coupled with the elastodynamics of multi-layered, composite structures, such as arterial walls, and with the flow of an incompressible, viscous fluid, such as blood. Examples showing the interaction between blood flow and stented coronary arteries sitting on a beating heart will be shown, and optimal design of stents for certain vascular procedures will be discussed.
The research presented in this talk was produced through an interdisciplinary collaboration between mathematicians (M. Bukac (Notre Dame), R. Glowinski (UH), J. Tambaca (Zagreb), Wang (UH)), and cardiovascular specialists from the Texas Medical Center in Houston (Dr. S. Little (Methodist Hospital Houston), Dr. W. Zoghbi (Methodist Hospital), Dr. D. Paniagua (Texas Heart Institute).
William R. Cannon
Systems Biology
Pacific Northwestern National Laboratory
"Multiscale Systems Biology: Thermodynamic Methods for Prediction of Cellular Dynamics, Metabolite Levels and Phenotypes"
Mass action dynamics are traditionally modeled with equations based on kinetic rate laws. However, thermodynamics also describes mass action phenomena and can be considered the original data analytics field. In this talk we will describe the complementary formulations of kinetics and thermodynamics and how a thermodynamic perspective provides insight that enables the modeling of cellular states and dynamics in complete agreement with kinetic rate laws and allows for a physically-principled approach to integrating systems biology data. We will also discuss the prediction of rate constants, reaction rates and metabolite levels from thermodynamic principles and the application of these methods to model the 24-hour circadian cycle of Neurospora spora, a model organism for circadian clocks, which are increasingly implicated in many diseases.
- Cannon, W. R. Simulating metabolism with statistical thermodynamics, PLoS One, vol. 9, p. e103582, 2014, DOI: 10.1371/journal.pone.0103582.
- Thomas, D. G., Jaramillo-Riveri, S., Baxter, D. J. and Cannon, W. R., Comparison of Optimal Thermodynamic Models of the Tricarboxylic Acid Cycle from Heterotrophs, Cyanobacteria and Green Sulfur Bacteria, J. Phys. Chem., 2014, DOI: 10.1021/jp5075913.
- Cannon, W. R. and Baker, S. E., Non-Steady State Mass Action Dynamics Without Rate Constants: Dynamics of Coupled Reactions using Chemical Potentials. 2017 Phys. Biol. 14/ DOI: 10.1088/1478-3975/aa7d80.
Jason M. Haugh
Chemical & Biomolecular Engineering
North Carolina State University
“Multi-scale modeling of wound healing: progress and challenges in modeling directed cell migration”
Chronic wounds are a major threat to public health and present as a comorbid complication with major diseases in humans. Although the proper healing of cutaneous wounds requires collective and coordinated behaviors of multiple cell types, a critical step is the recruitment and function of dermal fibroblasts, which are directed to invade the wound by gradients of a chemoattractant, platelet-derived growth factor (PDGF). A handful of biologicals, most notably recombinant PDGF-BB, are currently approved for treatment of wounds; however, the current treatments lack efficacy in accelerating wound healing, and consequently they have not gained traction in the clinic. These disappointing results underscore how poorly the dynamics of wound healing are understood at the tissue scale and the need to connect knowledge of molecular, cellular, and tissue-level processes to inform and predict outcomes of therapeutic strategies aimed at improving the rate and fidelity of wound repair. We have been developing models of fibroblast chemotaxis with consideration of molecular (polarization of signal transduction), supramolecular (assembly of actomyosin structures), cellular (biased cell movement), and tissue-level (wound invasion) dynamics, which span disparate time (seconds to weeks) and spatial (nm to cm) scales. In this talk, I will focus on the challenges of linking intracellular processes to macroscopic cell behavior and characterizing/modeling the diversity and heterogeneity of chemical and mechanical cues that bias cell movements in wounds.
- https://www.ncbi.nlm.nih.gov/pubmed/28700916
- https://www.ncbi.nlm.nih.gov/pubmed/25904526
- https://www.ncbi.nlm.nih.gov/pubmed/25482883
C. Anthony Hunt
Department of Bioengineering and Therapeutic Sciences
Schools of Pharmacy and Medicine University of California, San Francisco
“Scientifically Productive Virtual Experiments"
I will highlight recent progress (including problems and impediments that we have encountered, along with some lessons learned) in refining virtual experiment methods to achieve five objectives. 1) Discover and/or improve mechanism-based explanations for medically relevant phenomena of acetaminophen-induced liver injury, AILI. 2) Challenge competing mechanism-based hypotheses that are difficult or infeasible to challenge using wet-lab or clinical experiments. 3) Enable mechanism-based translation from one wet-lab system to another. Examples include [in vitro ⇔ rodent ⇔ human], [normal organ ⇔ diseased/damaged organ], and [± a (pharmacological) intervention]. 4) Provide plausible explanations for in vivo–in vitro disconnects at the phenomenon level. 5) Provide plausible explanations of intra- and interindividual variability at the phenomenon levels in terms of variability and/or heterogeneity at the mechanism level. The AILI research domain is well-suited for developing methods to achieve all five objectives. AILI is a frequently used model of liver disease onset, progression, and recovery. AILI is the most widely studied example of drug-induced liver injury (DILI), which is a significant barrier in development of new drugs and new therapeutic combinations. AILI in mice is the accepted model for AILI in humans. There is ample wet-lab data at all levels of mechanism-focused research (from genomic to whole organism). There are troublesome in vivo–in vitro disconnects spanning multiple phenomena that are barriers to translation. For most AILI phenomena, the considerable intra- and interindividual variability (in animals and humans) is poorly understood. Finally, there is mounting evidence of mechanism variability and heterogeneity among in vitro models used to study AILI and DILI.
George Karniadakis
Division of Applied Mathematics
Brown University
"Stochastic Multiscale Modeling of Hematological Disorders"
Modeling of hematological diseases, both genetic and infectious, such as anemias, elliptocytosis, diabetes, malaria, etc, requires a bottom-up approach to account for possible genetic mutations, cellular reorganization and adhesive dunamics. Molecular dynamics is ideal in this respect but it taxes computational resources heavily and cannot capture long-time integration or spatial scales of tens of microns relevant to such diseases. We have developed a new approach to multiscale modeling of complex systems using the dissipative particle dynamics (DPD) method, which is a coarse-grained version of molecular dynamics (MD) allowing simulations on larger domains and longer time scales than MD. We have also developed proper domain decomposition algorithms interfacing DPD with MD and the continuum Navier-Stokes equations for multiscale simulations across many orders of magnitude in spatio-temporal scales. Examples will be shown from coarse-graining red blood cells in health and diseases, including modeling of blood diseases such as malaria, diabetes, and sickle cell anemia. The correct governing equations of DPD will be derived based on the Mori-Zwanzig formulation including formulations with correlated structures (memory effects) and efficient algorithms to compute them.
- Y. Tang, L. Lu, H. Li, C. Evangelinos, L. Grinberg, V. Sachdeva, G. E. Karniadakis, "OpenRBC: A Fast Simulator of Red Blood Cells at Protein Resolution." Biophysical Journal, 2017, 112:2030-2037.
- Z. Li, H.S. Lee, E. Darve, G.E. Karniadakis, "Computing the non-Markovian coarse-grained interactions derived from the Mori-Zwanzig formalism in molecular systems: Application to polymer melts." J. Chem. Phys. 2017, 146, DOI:10.1063/1.4973347
- H. Chang, X. Li, H. Li, G.E. Karniadakis, "MD/DPD Multiscale framework for predicting morphology and stresses of red blood cells in health and disease." PLOS Computational Biology, 2016 DOI:10.1371/journal.pcbi.1005173.
Denise Kirschner
Department of Microbiology and Immunology
University of Michigan Medical School
"Tools for Building and Analyzing Multi-Scale models: TB as a Case Study"
Multi-scale models (MSM) are increasingly being used to study complex biological processes. Multi-scale models span a range of both spatial and temporal scales and can also encompass multiple physiological compartments. Multi-scale models are growing larger and more cumbersome and it is necessary to coarse grain model aspects when appropriate. A new approach that we call tuneable resolution can provide that flexibility. Tuneable resolution involves fine- or coarse-graining existing multi-scale models at the user’s discretion, allowing adjustment of the level of resolution specific to a question, an experiment, or a scale of interest. Tuneable resolution expands options for revising and validating mechanistic multi-scale models, can extend the longevity of multi-scale models, and may increase computational efficiency. The tuneable resolution approach can be applied to many model types, including differential equation, agent-based, and hybrid models and can be automated. Additionally, analyses of these models can be difficult, and we have fine-tuned a global uncertainty and sensitivity analysis approach that can be applied to all MSM types performing both inter- and intra-scale analyses. Finally, we have been exploring optimization, for example of drug treatment regimens, in the context of MSMs and have identified protocols that are computationally efficient.
Nathan Lewis
Department of Pediatrics
University of California, San Diego
"Capturing a more accurate view of tissue and cell-type specific metabolism"
Genome-scale models of metabolism aim to capture the activities of thousands of enzymes in a single computational model, and these can illuminate the molecular basis of cell phenotypes in healthy and diseased states. Such modeling promises to predict metabolic fluxes, metabolic limitations, and druggable targets in disease states. However, some enzymes are only active in specific cell types. To capture a more accurate view of cell type or tissue-specific metabolism, several algorithms have been developed. These methods use omics data to identify which genes are expressed and then use these to identify which pathways are active. However, these methods are often not rigorously benchmarked, and it is unclear how the selection of which algorithm or their associated parameters (e.g., gene expression thresholds, metabolic constraints) affect model content and predictive accuracy. To investigate this, we built hundreds of models of four different cancer cell lines using multiple algorithms, variations in gene expression thresholds, and different assumptions of model parameterization. The pathways that were predicted to active in the different cell lines varied substantially across different parameter sets. Despite this variability, the algorithms generally increased accuracy in predicting metabolic vulnerabilities and gene essentiality in the cancer cell lines. However, the selection of algorithm had the largest impact on model accuracy. In this talk I will further describe how assumptions made during model development influence model prediction accuracy. These insights will guide further development of context-specific models, thus enabling the development of more accurate models for diverse applications in biomedical research.
- Opdam, S., Richelle, A., Kellman, B., Li, S., Zielinski, D.C., Lewis, N.E. A systematic evaluation of methods for tailoring genome-scale metabolic models. Cell Systems, 4:1-12 (2017). DOI:10.1016/j.cels.2017.01.010
- Lewis, N.E., Schramm, G., Bordbar, A., Schellenberger, J., Andersen, M.P., Cheng, J.K., Patel, N., Yee, A., Lewis, R.A., Eils, R., König, R., Palsson, B.Ø. Large-scale in silico modeling of metabolic interactions between cell types in the human brain. Nature Biotechnology, 28:1279–1285 (2010).
- Kumar, A., Harrelson, T., Lewis, N.E., Gallagher, E., LeRoith, D., Shiloach, J., Betenbaugh, M.J.Multi-tissue computational modeling analyzes pathophysiology of Type 2 Diabetes in MKR mice. PLoS One, 9(7): e102319. DOI: 10.1371/journal.pone.0102319
Alison Marsden
Department of Pediatrics – Cardiology
School of Medicine
Department of Bioengineering
Stanford University
“Computational Investigations of the Biomechanical Underpinnings of Vein Graft Failure”
Coronary bypass graft surgery (CABG) is performed on approximately 500,000 patients every year in the United States. Because most patients require multi-vessel revascularization, roughly 70% of CABG surgeries employ saphenous vein grafts, despite the superior performance of arterial grafts. Vein graft failure continues to be a major clinical problem, with as many as 50% of grafts failing within 5 years of surgery. When a vein graft is implanted in the arterial system it adapts to the high flow and pressure of the arterial environment by changing composition and geometry. Though hemodynamics is known to play an active role in growth and remodeling of blood vessels, the underlying mechanisms of vein graft failure remain poorly understood. We will describe our two-pronged approach to investigating the biomechanical underpinnings of vein graft failure following CABG. First, we perform patient-specific simulations of coronary and bypass graft hemodynamics to compare the biomechanical forces acting on venous and arterial grafts. We will present recent advances in computational methodology in which we employ multiscale modeling methods to couple closed loop lumped parameter models of the coronary physiology to 3D hemodynamics simulations with fluid structure interaction and variable material properties. Second, we adapt a constrained mixture theory of growth and remodeling for use in vein grafts, and explore potential causes and amelioration of vein graft failure. Parameter estimation in these models is accelerated via optimization. Finally, we present a Bayesian framework for uncertainty quantification combining automated parameter estimation for clinical data assimilation and efficient multi-resolution expansion for uncertainty propagation to simulation predictions.
References:
- Ramachandra, A. B., Humphrey, J. D., Marsden, A. L., “Gradual loading ameliorates maladaptation in computational simulations of vein graft growth and remodeling,” Journal of the Royal Society Interface, Vol. 14 (130), May 2017.
- Schiavazzi, D. E., Doostan, A., Iaccarino, G., Marsden, A. L., “A Generalized Multi-resolution Expansion for Uncertainty Propagation,” Computer Methods in Applied Mechanics and Engineering, Vol. 314, pp. 196221, (2017).
- Tran, J. S., Schiavazzi, D. E., Ramachandra, A. B., Kahn, A. M., Marsden, A. L., “Automated Tuning for Parameter Identification in Multiscale Coronary Simulations,” Computers and Fluids, Vol. 142, pp. 128138, (2017).
- Ramachandra, A. B., Kahn, A. M., Marsden, A. L., “Patient specific simulations reveal significant differences in mechanical stimuli in venous and arterial coronary grafts,” Journal of Translational Cardiovascular Research, Vol. 9(4) pp. 279-290, (2016).
Andrew D. McCulloch
Department of Bioengineering
Department of Medicine School of Medicine
University of California San Diego
“Multi-Scale Modeling and Systems Mechanobiology of Ventricular Hypertrophy and Failure”
As many as 50% of patients with dilated cardiomyopathy have the additional complication of a ventricular electrical conduction defect. The prognosis of these patients with dyssynchronous heart failure is especially poor. 20 years ago, cardiologists discovered that cardiac resynchronization therapy (CRT) using biventricular electrical pacing can improve electromechanical dyssynchrony, stimulate reversal of heart failure remodeling, improve quality of life and prolong survival. However, 30-40% of patients undergoing CRT do not respond to the therapy. We have developed patient-specific models of ventricular electromechanics and seen that specific physiological quantities computed from these simulations, that change acutely when the pacemaker is turned on but can not be measured directly, appear to predict long-term responses after 6 months of therapy surprisingly well. These findings suggest that mechanoenergetic alterations in the myocardium caused by electromechanical dyssynchrony and reversed by the therapy may be signals for heart failure remodeling. We are now using biomechanical growth and remodeling laws to extend these models in time to predict the long-term response directly. However, these mechanisms are governed by multiple signaling pathways that are also modulated by heart failure and heart failure therapies. Therefore the long-term goal is to develop patient-specific models that are based on cell mechanosignaling and gene expression mechanisms and can predict the response of the failing heart to device and medical therapies.
Supported by NIH grants: P41 GM103426, R01 HL105242, R01 HL121754, U01 HL122199, U01 HL126273, U54 HL119893, and R01 HL137100
Eric Sobie
Icahn Medical Institute
Mount Sinai School of Medicine, New York, NY
"Exploiting mathematical models to predict differences between individuals in cardiac physiology”
Quantitative mismatches between human physiology and experimental models can present serious limitations for the development of effective therapeutics. We addressed this issue, in the context of cardiac electrophysiology, through mechanistic mathematical modeling combined with statistical analyses. Physiological metrics were simulated in heterogeneous populations describing cardiac myocytes from adult ventricles and those derived from induced pluripotent stem cells (iPSC-CMs). These simulated measures were used to construct a cross-cell type regression model that predicts adult myocyte drug responses from iPSC-CM behaviors. We found that quantitatively accurate predictions of responses to selective or non-selective drugs could be generated based on iPSC-CM responses and that the method can be extended to predict drug responses in diseased as well as healthy cells. This cross-cell type model can be of great value in drug development, and the approach, which can be applied to other fields, represents an important strategy for overcoming experimental model limitations.
- Devenyi et al, Journal of Physiology, 2017,
http://onlinelibrary.wiley.com/doi/10.1113/JP273191/abstract;jsessionid=9A3E2AFF82FB1DF26287281CA4011BA9.f02t01 - Lancaster and Sobie, Clinical Pharmacology & Therapeutics, 2016, http://onlinelibrary.wiley.com/doi/10.1002/cpt.367/abstract
- Cummins et al, PLOS Computational Biology, 2014, https://www.ncbi.nlm.nih.gov/pubmed/24675446
Melissa L. Knothe Tate
Biomedical Engineering, University of New South Wales, Australia
"Translation of Engineering Innovations Discovered through Multiscale, Coupled Imaging and Computational Modeling"
Through combination of novel microscopy protocols for imaging live cells and tissues as well as experimental mechanics methods, we have begun to elucidate mechanisms underpinning emergent properties of hierarchical materials such as tissues [1,2]. We refer to the process as Microscopy Aided Design And Manufacture (MADAMe). We apply this paired imaging and computational technology approach to engineer advanced materials that emulate the smart mechanical properties of tissues. These materials have applications in diverse arenas, from medical implants to the transport and sports industries. Our "bottom up" approach to emulating mechanically responsive natural materials integrates the fields of multiscale biomechanics and mechanobiology in novel ways and underscores the role of mechanics in life. It also elucidates how "brainless" cells adapt to dynamic mechanical environments by constantly weaving and thereby adapting their own niche [3]. In addition, our connectomics approach to understanding cell networks in situ, in tissues as diverse as brain and bone, provides a basis for a new approach to diagnostics, predicting emergent disease states using an epidemiological approach in cell populations within individual patients [3,5,6]. Challenges to the connectomics approach include acquisition, handling and archiving of massive data sets, discrepancies in technical capacities (e.g. resolution) of imaging methods, and hard and software approaches, as well as bridging and upskilling of research teams to apply a transdisciplinary approach using innovative conceptual, experimental, and translational approaches. This talk integrates our understanding of cells, expert tissue prototypers, and their networks, to emulating cellular approaches to engineer and manufacture materials and medical devices of the future.
[1] Knothe Tate ML (2017) Science/AAAS, A New Age in Scanning Electron Microscopy: Applications in the Life Sciences, pp. 19-23.
[2] Ng J et al. Sci Reports (2017) 7, 40396.
[3] Knothe Tate ML et al. Adv Healthcare Mat (2016) 5, 1581.
[4] Knothe Tate ML et al. BioArchitecture (2016) 6, 85.
[5] Eberle A-L et al. J Microscopy (2015) 259, 114.
[6] Pereira A et al. PLoS Comp Biol (2016) 12, e1005217.
John Weisel
Departments of Cell and Developmental Biology
University of Pennsylvania Perelman School of Medicine
"Multi-scale methods for studying blood clot and thrombus structure and mechanics from nanometers to patients"
An understanding of the biomechanics of hemostasis and thrombosis requires knowledge of a whole array of events from the submolecular level all the way up to patient pathophysiology. The structure and mechanical properties of blood clots are essential in vivo for the ability of clots to stop bleeding in flowing blood but also determine the likelihood of obstructive thrombi that cause heart attacks and strokes. Despite such critical importance, the molecular structural basis of clot mechanics is not well understood, as well as other aspects on all spatial scales. This talk will focus on recent biophysical research on both platelet aggregation and fibrin polymerization, as well as our collaborative multiscale modeling with Mark Alber and colleagues. We have studied the structures of components of blood clotting from the molecular and cellular levels, and whole clots and thrombi from patients. An optical trap system has been developed to study protein-protein binding/unbinding at the single molecule level, and used to characterize fibrin polymerization and fibrinogen-integrin interactions that are responsible for platelet aggregation. The results of this research are relevant to the behavior of blood clots in flowing blood. Through studies of the structure and mechanical behavior of clots at the macroscopic, network, fiber and molecular levels, we show that they can only be understood by integration of their materials properties at all these levels. We have also demonstrated the physical mechanism of clot contraction and showed platelet filopodia pulling on fibrin fibers to shrink the clot. The long-term goal is to relate these basic science discoveries to thrombotic disorders, bleeding, and embolization in coronary artery disease, stroke and cancer, as well as angioplasty and methods of clot ablation and removal, and application to fibrin sealants.
- References
- Tutwiler V, Peshkova AD, Andrianova IA, Khasanova DR, Weisel JW, Litvinov RI. Contraction of blood clots is impaired in acute ischemic stroke, Arteriosler. Thromb. Vasc. Biol. 37:271-279, 2017
- Kim OV, Litvinov RI, Alber MS, and Weisel JW. Quantitative structural mechanobiology of platelet-driven blood clot contraction, Nature Commun. 18:1274, 2017
- Xu S, Xu Z, Kim OV, Litvinov RI, Weisel JW, and Alber MS. Deformation, embolization and permeability of partially obstructive blood clots under shear flow, J. Roy. Soc. Interface, in press, 2017
Laurence Yang
Systems Biology Research Group
University of California, San Diego
"Expanding the biological scope of multi-scale models of Metabolism and protein Expression"
Predicting microbial behavior under various environments has relevance for the study of infectious disease and the human microbiomes. Genome-scale models (GEMs) are mathematical optimization-based models that predict fluxes through biochemical reaction networks comprising microbial metabolism. Since 2012, GEMs have been extended to include protein expression, resulting in multi-scale models of Metabolism and macromolecular Expression (ME models). In this talk, we present two aspects on the Methodology of ME models. Using a model of Escherichia coli, we first present how we overcame specific computational challenges for solving the ill-conditioned optimization problems associated with ME models1. Second, we present how we are further expanding the biological scope described by these models2, particularly for modeling proteostasis and response to physicochemical stresses3.
Relevant Publications
- L Yang, D Ma, A Ebrahim, CJ Lloyd, MA Saunders, BO Palsson (2016) BMC Bioinform 17:391
- L Yang, JT Yurkovich, CJ Lloyd, A Ebrahim, MA Saunders, BO Palsson (2016) Sci Rep 6:36734
- K Chen, Y Gao, N Mih, EJ O’Brien, L Yang, BO Palsson (2017) Proc Natl Acad Sci USA doi:10.1073/pnas.1705524114